Protocol-Based Clinical Trial Document Generation
Protocol-Based Clinical Trial Document Generation
Built for regulated clinical workflows. Designed for accuracy, compliance, and audit readiness.
Protocol-Based Clinical Trial Document Generation
Turn clinical trial protocols into structured, review-ready documents for Sites, CROs, and Sponsors - with full traceability to source data.
Built for regulated clinical workflows. Designed for accuracy, compliance and audit readiness.



Protocol-Based Clinical Trial Document Generation
Turn clinical trial protocols into structured, review-ready documents for Sites, CROs and Sponsors - with full traceability to source data.
Built for regulated clinical workflows. Designed for accuracy, compliance and audit readiness.
From Clinical Trial Protocols to Review-Ready Documents
Clinials reads your clinical trial protocol and supporting documents to generate accurate, role-specific clinical documentation used across feasibility, study operations, regulatory review, and patient communication.
Schedule of Activities

A complete Schedule of Activities from clinical trial protocols, including biomarkers, procedures, and endpoints organised by visit to help sites and sponsors plan study execution and budgets.
Operational Protocol Synopsis

A structured operational protocol synopsis summarising study design, objectives, endpoints, procedures, and operational requirements for study teams.
Budget Discovery

A protocol-derived breakdown of study activities, assessments, biomarkers, and endpoints to support feasibility and early budget planning.
Built for Clinical Trial Compliance and Data Security
Clinials is designed for regulated clinical trial environments, ensuring every generated document is secure, traceable to source data, and suitable for regulatory review, audits, and cross-functional collaboration.
Encrypted & Private
All clinical trial documents and source files are encrypted and remain within your organisation.
Regulatory Compliance
Designed to support GDPR, HIPAA, and regulated clinical trial workflows.
Controlled Access
Role-based permissions for sites, CROs, sponsors, and partners.
Versioning & Traceability
Track document versions and maintain clear links back to protocol source data.
“I have used Clinials on 3 studies now. The output is clean and very professional. I continually watch for updates as each one offers even more valuable features.
It allows our team to focus on other details knowing that these documents can be created seamlessly and quickly.”- Consultant for the medical research industry (USA)
“Super engaging and excellent platform.”
- CRA (USA)
“Where has this solution been? I’ve needed it forever. It’s amazing!”
- Site network Manager (USA)
“You have a great product that will only become increasingly powerful with continuous advancements in AI capabilities. I have a number of biotech clients and will discuss your solution with them.”
-Biotech advisor/consultant
Trusted by Clinical Trial Teams Worldwide:
Sites, CROs, Sponsors & Labs
Protocol-driven clinical documents for feasibility, operations, regulatory review, and patient communication.
Explore by Role
From Clinical Trial Protocols to Review-Ready Documents
Clinials reads your clinical trial protocol and supporting documents to generate accurate, role-specific clinical documentation used across feasibility, study operations, regulatory review, and patient communication.
Schedule of Activities

A complete Schedule of Activities from clinical trial protocols, including biomarkers, procedures, and endpoints organised by visit to help sites and sponsors plan study execution and budgets.
Operational Protocol Synopsis

A structured operational protocol synopsis summarising study design, objectives, endpoints, procedures, and operational requirements for study teams.
Budget Discovery

A protocol-derived breakdown of study activities, assessments, biomarkers, and endpoints to support feasibility and early budget planning.
Built for Clinical Trial Compliance and Data Security
Clinials is designed for regulated clinical trial environments, ensuring every generated document is secure, traceable to source data, and suitable for regulatory review, audits, and cross-functional collaboration.
Encrypted & Private
All clinical trial documents and source files are encrypted and remain within your organisation.
Regulatory Compliance
Designed to support GDPR, HIPAA, and regulated clinical trial workflows.
Controlled Access
Role-based permissions for sites, CROs, sponsors, and partners.
Versioning & Traceability
Track document versions and maintain clear links back to protocol source data.
How Clinials Turns a Clinical Trial Protocol Into Review-Ready Documents
Clinials transforms your clinical trial protocol into structured, traceable documents used across feasibility, operations, regulatory review, and patient communication.

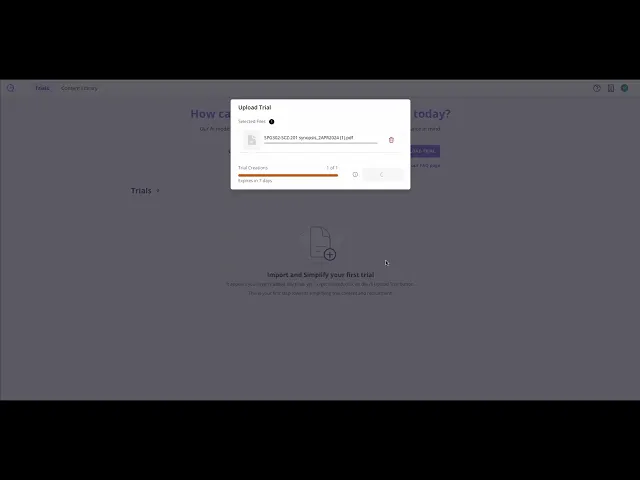
Step 1: Upload Protocol
Upload your protocol and supporting documents (e.g. Schedule of Activities, Investigator Brochure). Clinials securely ingests and structures protocol content.
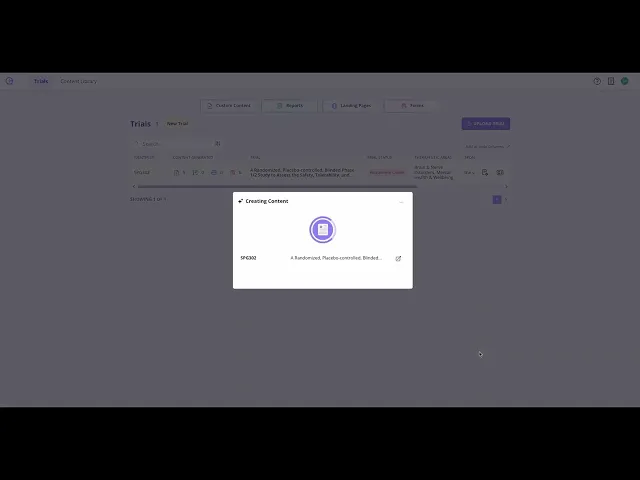
Step 2: Create Content
Choose the documents you need — from patient information sheets and operational summaries to budget discovery and feasibility reports.
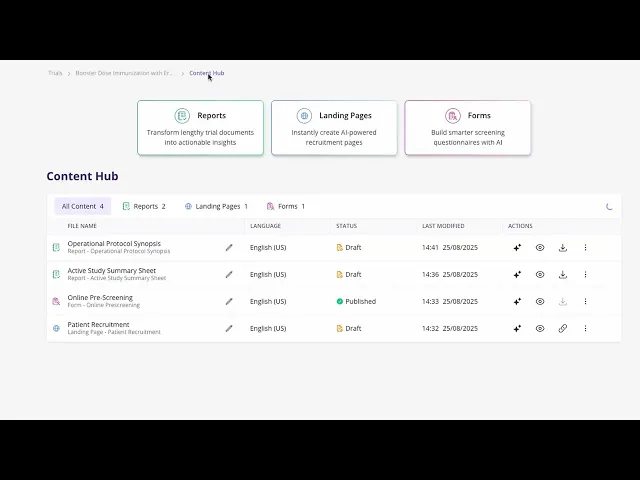
Step 3: Communicate your Trial
Manage your content! This includes:
Edit and Previewing
Versioning Control
Download in PDF, DOCX and CSV
Every output is grounded in your source documents, fully traceable, and ready for review, collaboration, and export.
Trusted by Clinical Trial Teams Worldwide
Protocol-driven clinical documents for feasibility, operations, regulatory review, and patient communication.
“I have used Clinials on 3 studies now. The output is clean and very professional. I continually watch for updates as each one offers even more valuable features.
It allows our team to focus on other details knowing that these documents can be created seamlessly and quickly.”- Consultant for the medical research industry (USA)
“Super engaging and excellent platform.”
- CRA (USA)
“Where has this solution been? I’ve needed it forever. It’s amazing!”
- Site network Manager (USA)
“I think you have a great product that will only become increasingly powerful with continuous advancements in AI capabilities.
I have a number of biotech clients and will discuss your solution with them.”- Biotech advisor/consultant (Australia)
Built for Clinical Trial Compliance and Data Security
Clinials is designed for regulated clinical trial environments, ensuring every generated document is secure, traceable to source data, and suitable for regulatory review, audits, and cross-functional collaboration.
Encrypted & Private
All clinical trial documents and source files are encrypted and remain within your organisation.
Regulatory Compliance
Designed to support GDPR, HIPAA, and regulated clinical trial workflows.
Controlled Access
Role-based permissions for sites, CROs, sponsors, and partners.
Versioning & Traceability
Track document versions and maintain clear links back to protocol source data.
See How Clinials Generates Review-Ready Clinical Trial Documents
Trusted by Clinical Trial Teams Worldwide:
Sites, CROs, Sponsors & Labs
Protocol-driven clinical documents for feasibility, operations, regulatory review, and patient communication.
Explore by Role
“I have used Clinials on 3 studies now. The output is clean and very professional. I continually watch for updates as each one offers even more valuable features.
It allows our team to focus on other details knowing that these documents can be created seamlessly and quickly.”- Consultant for the medical research industry (USA)
“Super engaging and excellent platform.”
- CRA (USA)
“Where has this solution been? I’ve needed it forever. It’s amazing!”
- Site network Manager (USA)
“You have a great product that will only become increasingly powerful with continuous advancements in AI capabilities. I have a number of biotech clients and will discuss your solution with them.”
-Biotech advisor/consultant
How Clinials Turns a Clinical Trial Protocol Into Review-Ready Documents
Clinials transforms your clinical trial protocol into structured, traceable documents used across feasibility, operations, regulatory review, and patient communication.

Step 1: Upload Protocol
Upload your protocol and supporting documents (e.g. Schedule of Activities, Investigator Brochure). Clinials securely ingests and structures protocol content.

Step 2: Create Content
Choose the documents you need — from patient information sheets and operational summaries to budget discovery and feasibility reports.

Step 3: Communicate your Trial
Manage your content! This includes:
Edit and Previewing
Versioning Control
Download in PDF, DOCX and CSV
Every output is grounded in your source documents, fully traceable, and ready for review, collaboration, and export.
How Clinials Turns a Clinical Trial Protocol Into Review-Ready Documents
Clinials transforms your clinical trial protocol into structured, traceable documents used across feasibility, operations, regulatory review, and patient communication.

Step 1: Upload Protocol
Upload your protocol and supporting documents (e.g. Schedule of Activities, Investigator Brochure). Clinials securely ingests and structures protocol content.

Step 2: Create Content
Choose the documents you need — from patient information sheets and operational summaries to budget discovery and feasibility reports.

Step 3: Communicate your Trial
Manage your content! This includes:
Edit and Previewing
Versioning Control
Download in PDF, DOCX and CSV
Every output is grounded in your source documents, fully traceable, and ready for review, collaboration, and export.
See How Clinials Generates Review-Ready Clinical Trial Documents
See How Clinials Generates Review-Ready Clinical Trial Documents
Built for Clinical Trial Compliance and Data Security
Clinials is designed for regulated clinical trial environments, ensuring every generated document is secure, traceable to source data, and suitable for regulatory review, audits, and cross-functional collaboration.
Encrypted & Private
All clinical trial documents and source files are encrypted and remain within your organisation.
Regulatory Compliance
Designed to support GDPR, HIPAA, and regulated clinical trial workflows.
Controlled Access
Role-based permissions for sites, CROs, sponsors, and partners.
Versioning & Traceability
Track document versions and maintain clear links back to protocol source data.
“I have used Clinials on 3 studies now. The output is clean and very professional. I continually watch for updates as each one offers even more valuable features.
It allows our team to focus on other details knowing that these documents can be created seamlessly and quickly.”- Consultant for the medical research industry (USA)
“Super engaging and excellent platform.”
- CRA (USA)
“Where has this solution been? I’ve needed it forever. It’s amazing!”
- Site network Manager (USA)
“You have a great product that will only become increasingly powerful with continuous advancements in AI capabilities. I have a number of biotech clients and will discuss your solution with them.”
-Biotech advisor/consultant
Trusted by Clinical Trial Teams Worldwide:
Sites, CROs, Sponsors & Labs
Protocol-driven clinical documents for feasibility, operations, regulatory review, and patient communication.
Explore by Role
How Clinials Turns a Clinical Trial Protocol Into Review-Ready Documents
Clinials transforms your clinical trial protocol into structured, traceable documents used across feasibility, operations, regulatory review, and patient communication.

Step 1: Upload Protocol
Upload your protocol and supporting documents (e.g. Schedule of Activities, Investigator Brochure). Clinials securely ingests and structures protocol content.

Step 2: Create Content
Choose the documents you need — from patient information sheets and operational summaries to budget discovery and feasibility reports.

Step 3: Communicate your Trial
Manage your content! This includes:
Edit and Previewing
Versioning Control
Download in PDF, DOCX and CSV
Every output is grounded in your source documents, fully traceable, and ready for review, collaboration, and export.
See How Clinials Generates Review-Ready Clinical Trial Documents
From Clinical Trial Protocols to Review-Ready Documents
Clinials reads your clinical trial protocol and supporting documents to generate accurate, role-specific clinical documentation used across feasibility, study operations, regulatory review, and patient communication.
Schedule of Activities

A complete Schedule of Activities from clinical trial protocols, including biomarkers, procedures, and endpoints organised by visit to help sites and sponsors plan study execution and budgets.
Operational Protocol Synopsis

A structured operational protocol synopsis summarising study design, objectives, endpoints, procedures, and operational requirements for study teams.
Budget Discovery

A protocol-derived breakdown of study activities, assessments, biomarkers, and endpoints to support feasibility and early budget planning.