
Upload your protocol, IB, or source documents, then generate plain-language summaries, patient content, and trial-ready materials, perfectly aligned and ready for clinical teams to use.
Key features
Protocol summaries
Create detailed yet digestible summaries tailored to diverse audiences.
Patient-friendly content
Transform complex documents into easy-to-understand materials for patients.
Multilingual content
Communicate with stakeholders from diffferent global regions and ethnicity.
Standardization
Save time with standardized formatting for content creation.
Multi-format
Different formats to suit audience type and communication needs.
Team branding
Boost user engagement and reinforce organizational identity.
From complexity to clarity in minutes!
Clinials turns your protocol and source documents into aligned, human-ready trial content: plain-language summaries, patient-ready text, recruitment copy, and operational outputs. Built for busy teams. Designed for real-world trials.
A range of protocol summaries to suit the needs of different teams
Empower your teams with tailored protocol summaries that enhance clarity, improve collaboration, and support faster decision-making across every stage of your trial.

Localized content in over 10 languages to strengthen your global operations
Bridge communication gaps with precise, multilingual content that fosters stronger partnerships, ensures consistency across global clinical operations, and enhances patient engagement.

Say goodbye to manual data sorting. Clinials pulls essential biomarkers and endpoints directly from trial documents, ensuring accuracy and immense time saving.
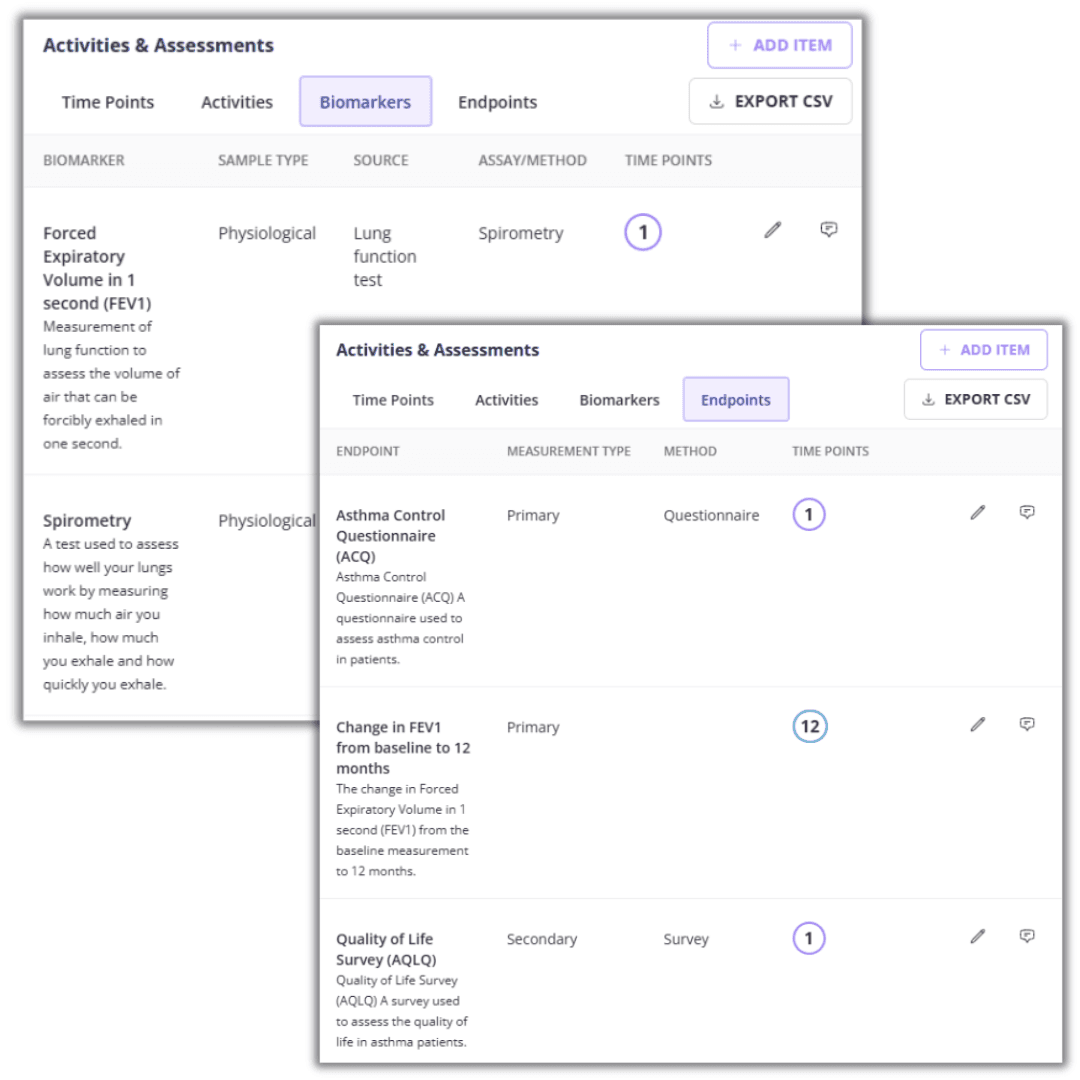
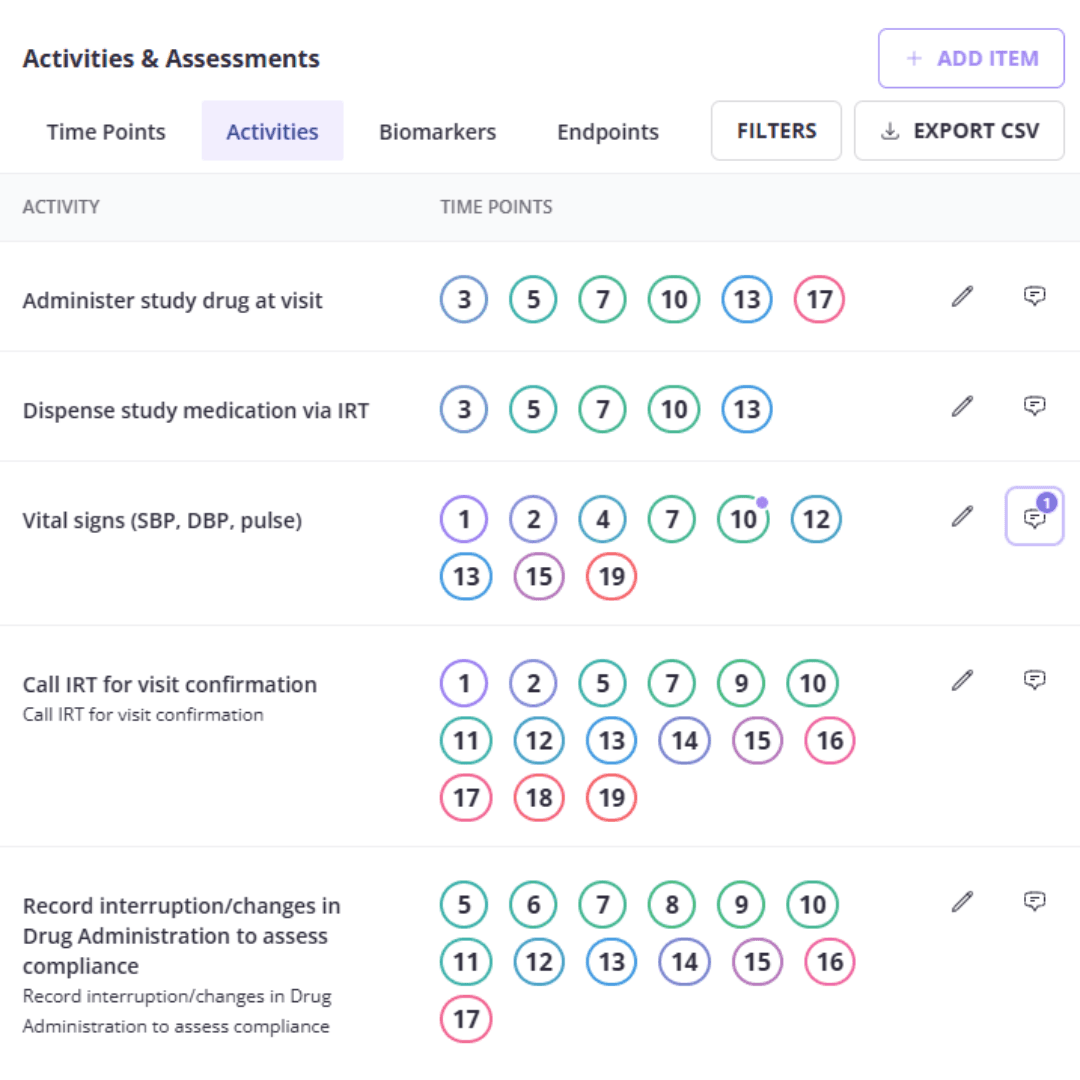
Organized and interactive schedule of actvities
Make data organization a breeze with easy-to-use SOA and endpoint summaries that also capture the footnotes; all editable and exportable.

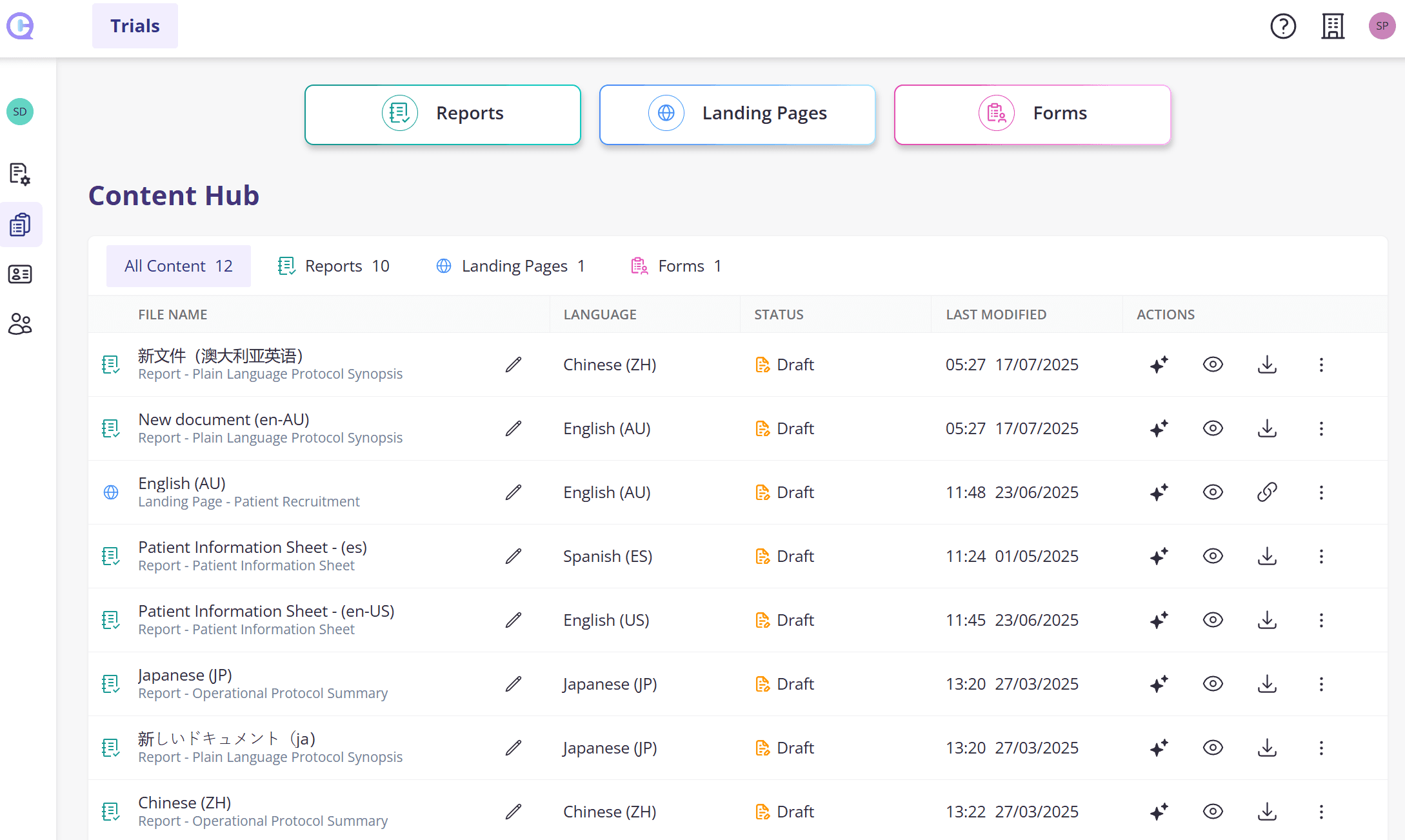
Create content that matters to you - Fast
A powerful and seamless experience to generate, view and edit various trial content.
Plain language protocol synopsis (PLPS)
Operational protocol summary (OPS)
Patient Information Sheet
Informational landing pages
Screening forms
Biomarkers and endpoints summary
Schedule of activities summary
Clinials AI in 4 steps
In mere minutes take your complex protocol or a clinicaltrials.gov listing and transform it into plain language or scientific based content. Our process ensures your protocol remains private and secure.
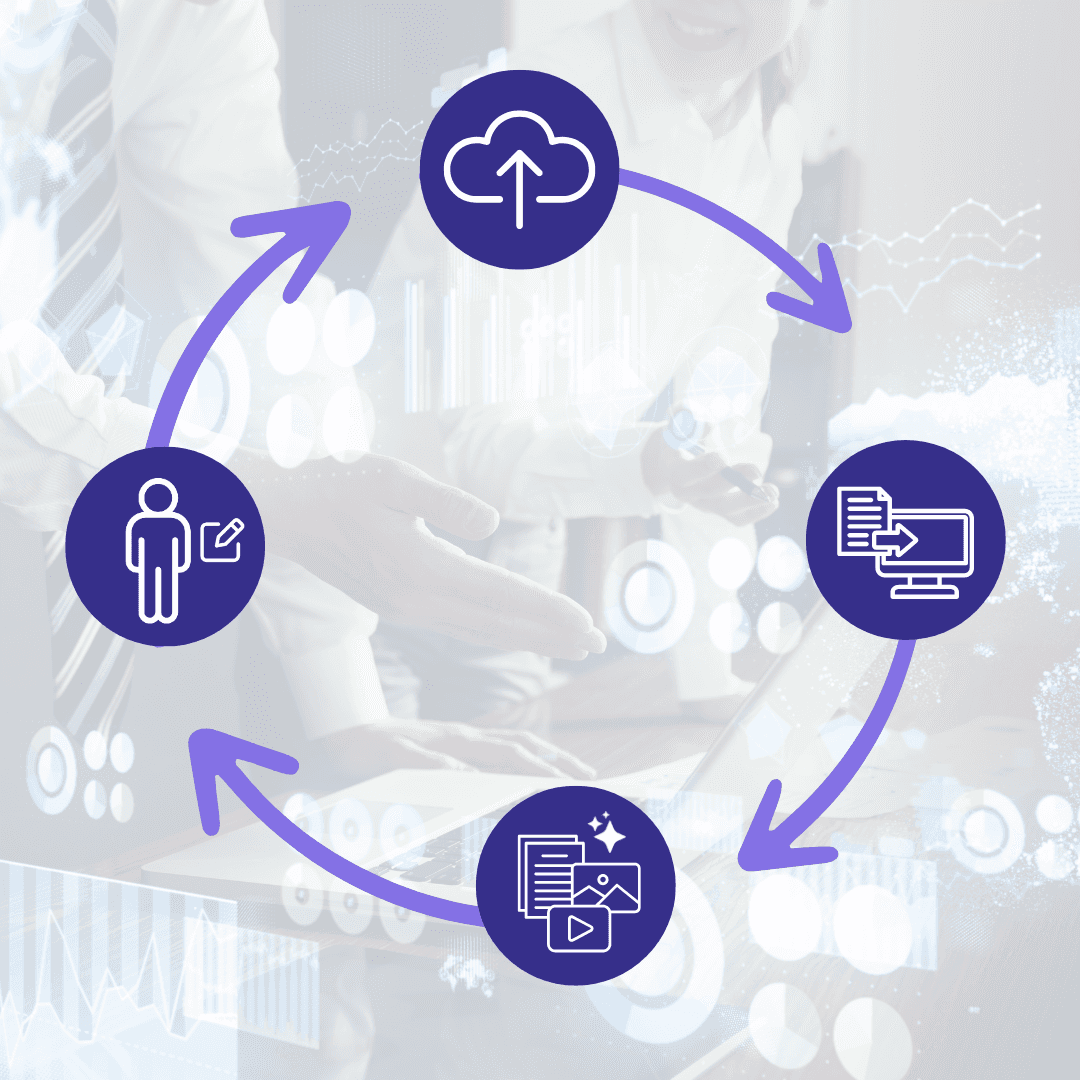
01
Upload your trial
Upload from a Trial Protocol or from a clinicaltrials.gov NCTID, includes bulk upload option.
02
Review digital protocol
Our platform will extract the necessary information for content creation.
03
Generate content
Let our AI turn your trial into content that matters to you.
04
Brand, edit and curate
Make any changes you like using our in-built editors, always a human-in-the-loop.