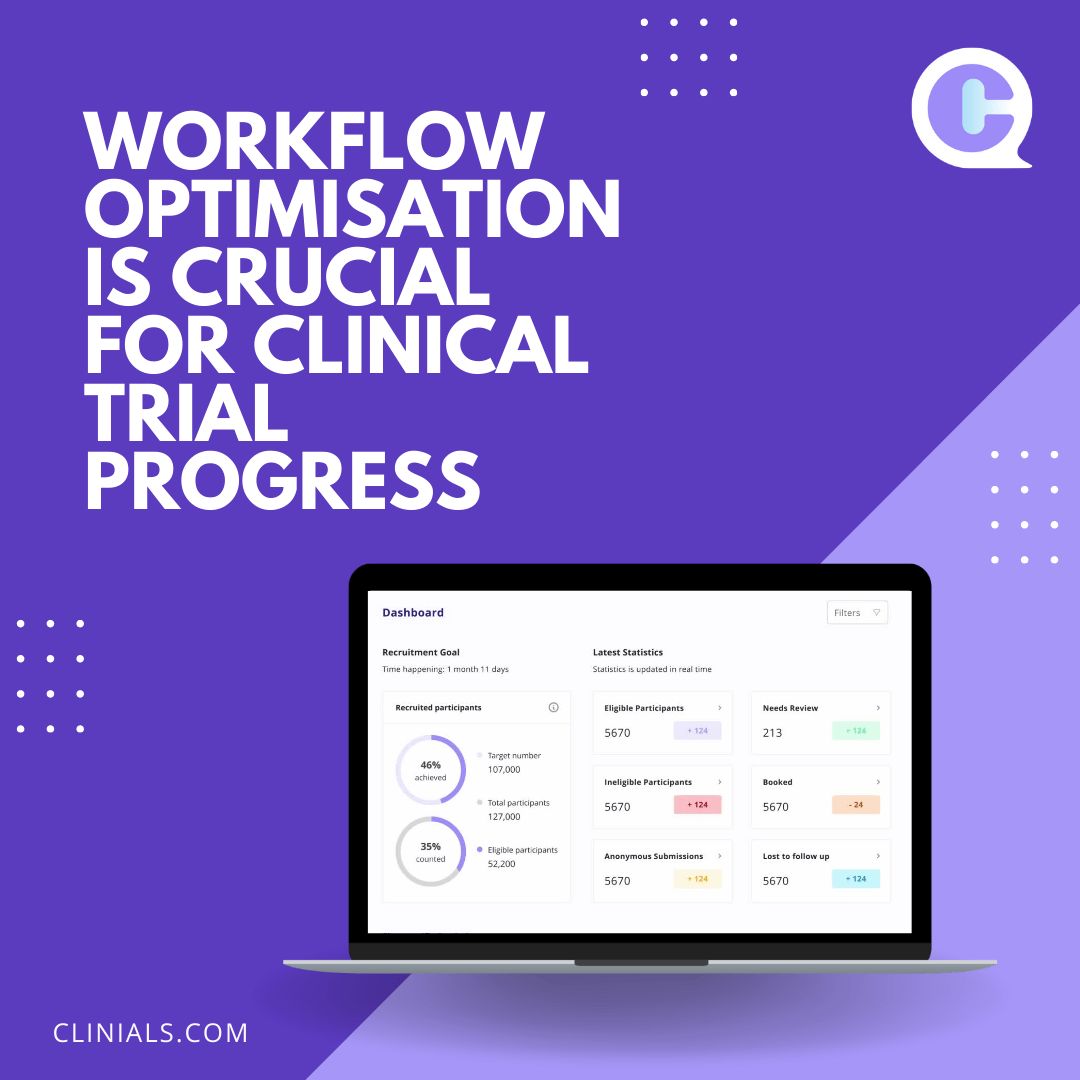
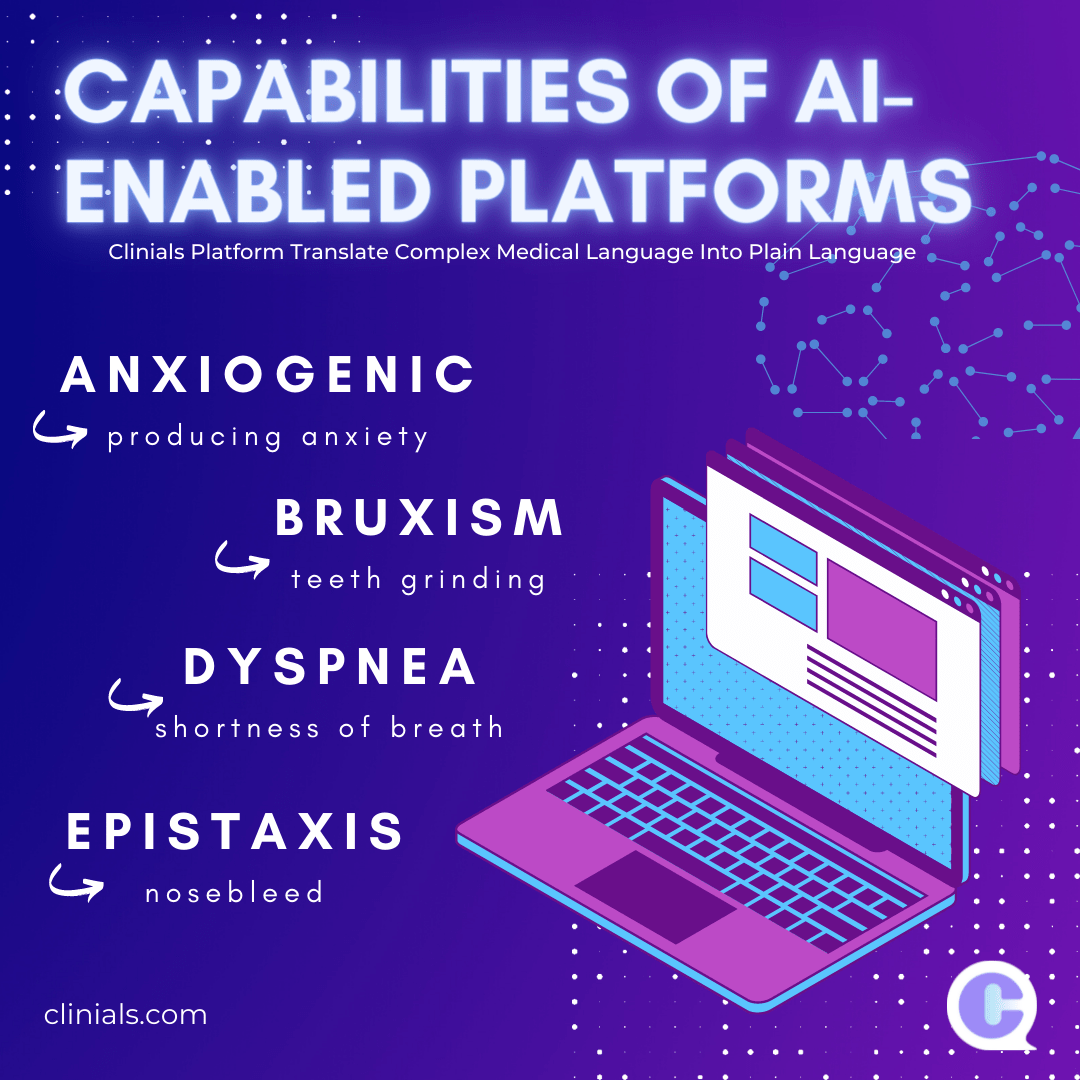
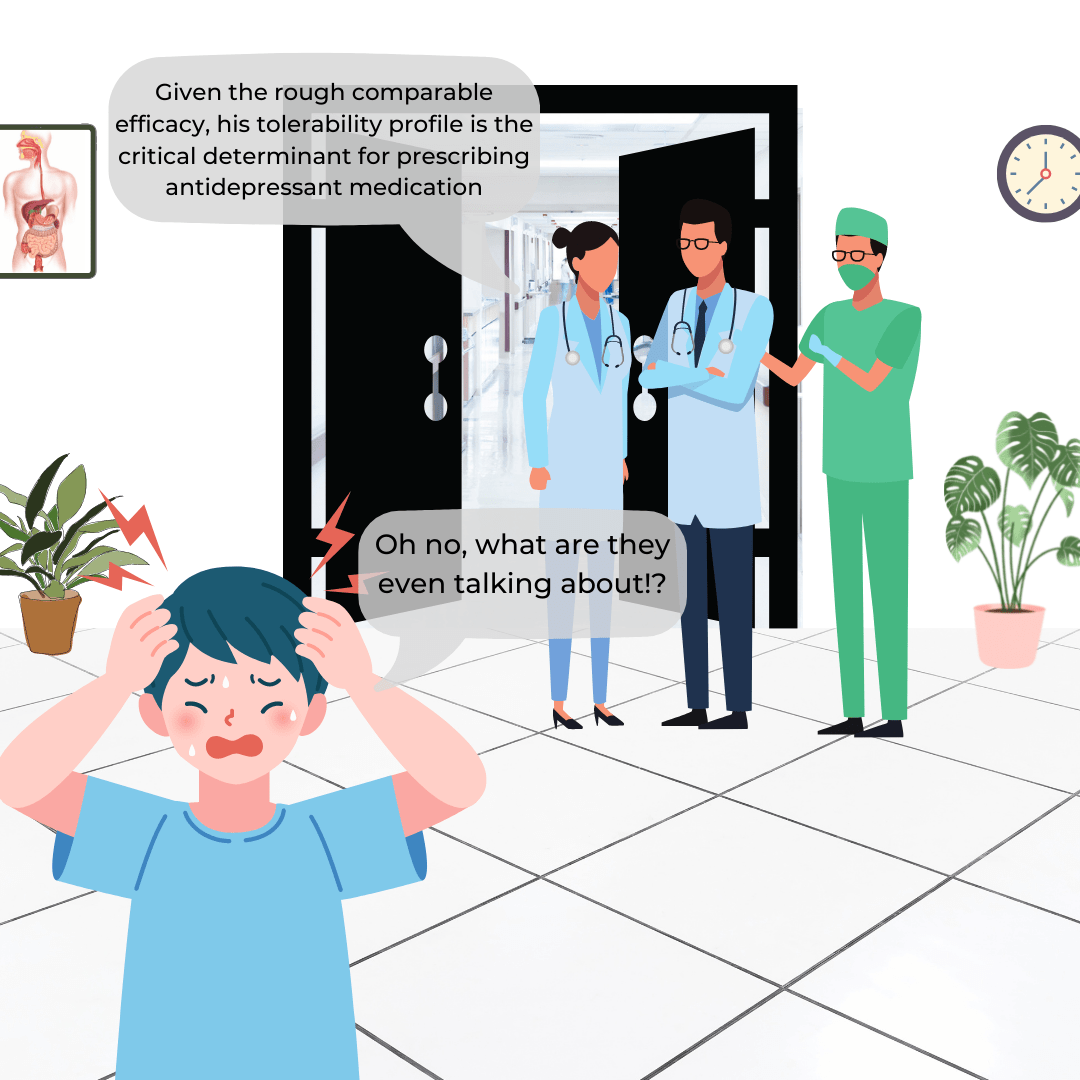
Clinical trials are costly necessities for research and development (R&D) of novel devices and treatments, yet with more than half failing to finish, they become even costlier for pharmaceutical companies. Without R&D, we would not have products like birth control or cancer interventions. To protect organizations financially that build the cures of the future, we… Continue reading The Hidden Costs and Consequences of Delaying a Clinical Trial
The Hidden Costs and Consequences of Delaying a Clinical Trial