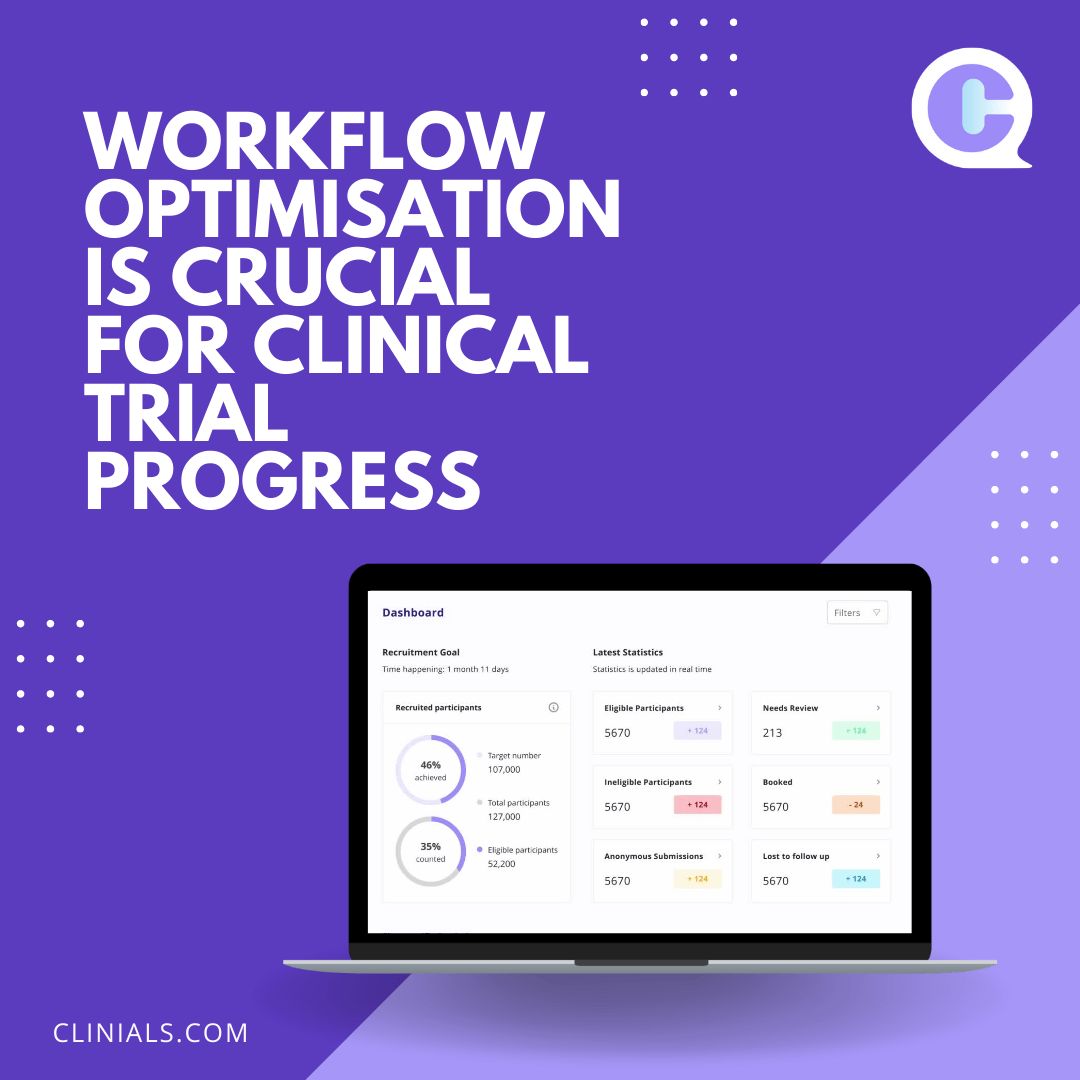
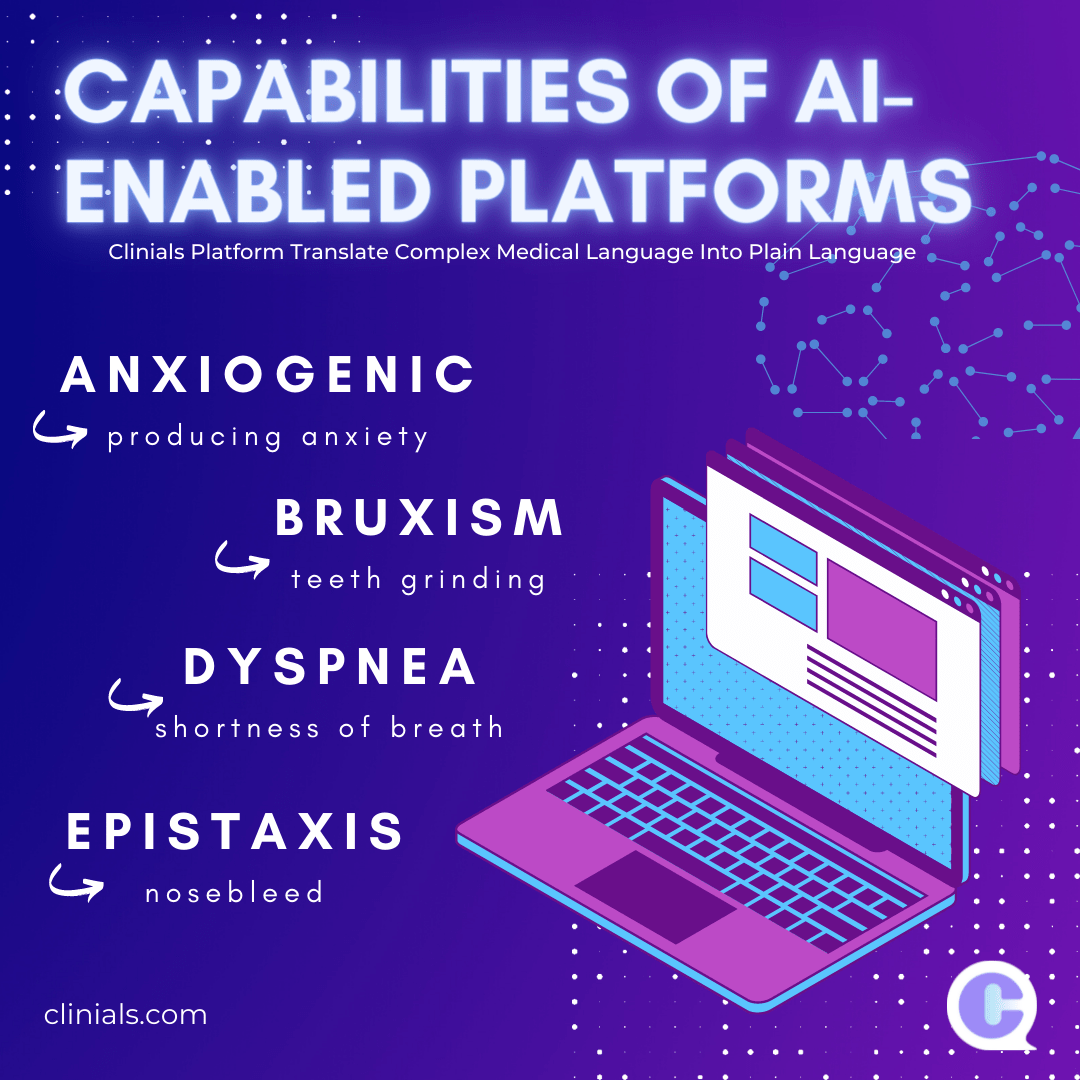
How can Biotechnology and Medtech device companies use AI to increase Diversity in their clinical trials? Global pharmaceutical companies will need to make use of AI’s transformative potential in addressing the diversity gap in clinical trials to illuminate a pathway towards more inclusive, accessible and patient-centric healthcare practices. Innovative biotechnology and Medtech device companies are… Continue reading Pharma to use AI to increase Diversity in their clinical trials
Pharma to use AI to increase Diversity in their clinical trials