

A Little About Us
Clinials is an innovative AI-enabled platform designed to simplify the process for patients and individuals to searching for clinical trials. It provides those interested in participating in clinical research with a easy to understand, user-friendly interface where they can easily find trials that match their specific criteria. The platform utilizes advanced language processing capabilities to transform complex medical jargon into plain language, making trial information more accessible and understandable to a more diverse audience.
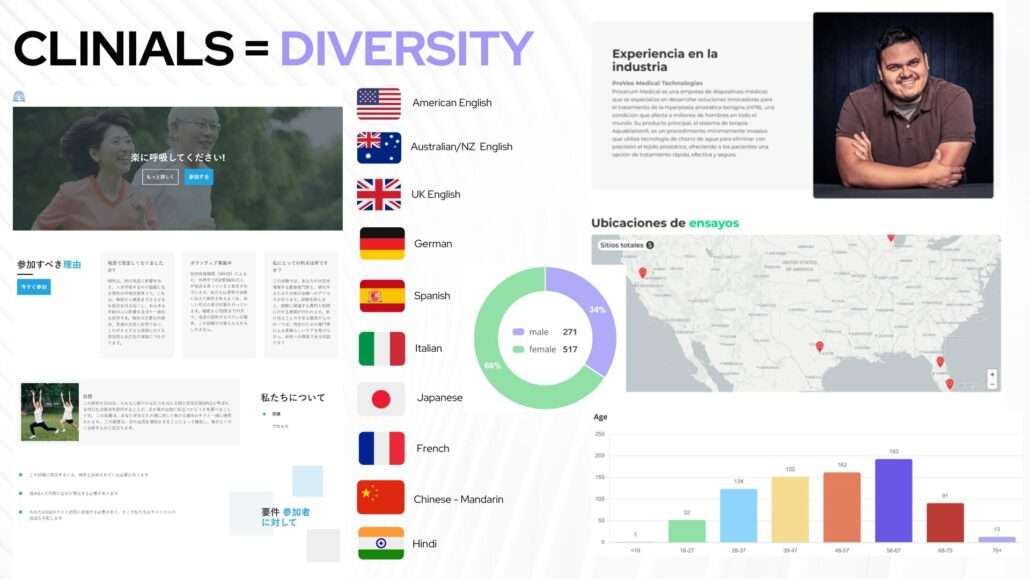
Unlocking Value: The Transformative Power of Diversity in Clinical Trials
In the fast-paced realm of clinical trials, where innovation meets the pursuit of groundbreaking therapies, there lies a critical bottleneck that demands our attention—the challenge of patient recruitment. A staggering 83% of clinical trials globally grapple with delays or failure due to recruitment hurdles, and it’s time to explore a strategic avenue that not only addresses this challenge but also unlocks unprecedented value: diversity.
The Economic Imperative of Diversity
Investors, listen closely. Beyond the moral imperative, embracing diversity in clinical trials presents a compelling economic case. Imagine a scenario where trials are completed faster, and market entry is accelerated. This isn’t just a theoretical advantage; it’s a tangible promise backed by a broader participant base that expedites the accumulation of valuable data.
Swift trial completion and increased market potential directly correlate with more attractive investments. We understand that reaching a diverse population comes with associated costs, but the benefits far outweigh them. Less time spent contracting trial sites, a larger customer base, and faster market entry—it’s a proposition that resonates with investors seeking not just returns but rapid, strategic returns.
Strategies for Cost-Efficiency and Enhanced Recruitment
Clinical trials often rely on databases that are site-based, leading to restricted recruitment pools and costly delays. We propose a paradigm shift—expanding the search beyond these databases to enlarge the overall potential patient pool. This isn’t just about efficiency; it’s about redefining the very landscape of clinical trial dynamics.
Cost-effective strategies for participant engagement take center stage, emphasizing efficiency for quicker trials and accelerated market access. We’re not merely discussing strategies; we’re reshaping the very architecture of clinical trial efficiency, making it a magnet for investor interest.


Communication and Readiness for Market Success
In the journey from the laboratory to the market, communication plays a pivotal role. Complex medical details often hinder recruitment efforts, impacting trial timelines and delaying progress. Streamlined communication isn’t just a tactical move; it’s a strategic choice to accelerate participant understanding and facilitate faster trials.
Quality Prescreening and the Economic Impact
Simplifying the prescreening process isn’t just about administrative ease; it’s about attracting a more intellectually diverse participant pool. This strategic move has direct economic repercussions—improved prescreening submissions lead to higher-quality participant data and increased patient retention. The result? An accelerated trial timeline bringing products to market faster with the potential to save trials that are unable to reach the recruitment goals.
Data-Driven Diversity and Inclusive Progress
“Diverse data not only measures success but enhances our insights.” This statement encapsulates the essence of our commitment to inclusivity. We acknowledge that we can’t change what we don’t track. Diverse data isn’t just a metric; it’s a dynamic force propelling us toward more inclusive and effective clinical trials.
Engagement Strategies for Rapid Market Entry
The dots connect seamlessly—participant engagement strategies directly contribute to increased recruitment efficiency and, consequently, faster market entry. For investors seeking not just returns but a shorter time to market, this is an economic advantage that resonates.
A Call to Action
In conclusion, we invite Investors, Sponsors, Clinical Research Organizations (CROs) and Site stakeholders to reach out to us for a chat to see how we can aid in making your clinical trial a success.
Consider that diversity is not just about culture but education and socioeconomics; it’s a strategic move that warrants action at the highest levels. The potential is immense—faster trials, quicker market access, and increased returns. By embracing diversity in clinical research, we not only contribute to individual trial success but position ourselves for quicker returns and increased market competitiveness.
